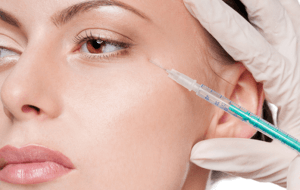
FDA Approves BOTOX® Cosmetic (onabotulinumtoxinA) for Temporary Improvement of Moderate to Severe Lateral Canthal Lines (Crow’s Feet Lines) in Adults
Newest Indication for Number-One Prescribed Facial Aesthetic Treatment in U.S. Can Help Improve Appearance of Lines Around the Eyes
IRVINE, Calif.–(BUSINESS WIRE)– Allergan, Inc., (NYSE: AGN) today announced approval by the U.S. Food and Drug Administration (FDA) to market BOTOX® Cosmetic (onabotulinumtoxinA), for an additional indication to temporarily treat moderate to severe lateral canthal lines, commonly known as “crow’s feet” lines. BOTOX® Cosmetic is the first and only product of its kind approved for this indication. BOTOX® Cosmetic, approved in the United States in 2002 for the temporary improvement of moderate to severe glabellar lines (frown lines between the brows) for patients aged 18 to 65 years, remains the number-one minimally invasive aesthetic medical treatment globally.
“Allergan has remained the leader in the facial aesthetic industry by prioritizing research and development that provides physicians and patients with innovative treatments and fully explores the additional indications for our product portfolio,” said Scott W. Whitcup M.D., Executive Vice President, Research and Development, Chief Scientific Officer, Allergan. “We are pleased that the FDA has approved a new indication for BOTOX® Cosmetic to temporarily improve the appearance of crow’s feet lines. With this approval, BOTOX® Cosmetic is now the only pharmaceutical approved to treat both crow’s feet lines and frown lines between brows. This approval will enhance our ability to work with and train aesthetic physicians on the science of administering BOTOX® Cosmetic to yield the best possible outcomes for patients.”
The safety and efficacy of BOTOX® Cosmetic as a treatment for crow’s feet lines was demonstrated in two randomized, multi-center, placebo-controlled clinical trials. The studies enrolled more than 1,350 subjects with 833 subjects receiving treatment with BOTOX® Cosmetic. The trial demonstrated that BOTOX® Cosmetic was an effective treatment compared to the control group, which did not receive BOTOX® Cosmetic treatment.
“Crow’s feet lines are defined as the lines that extend around the corner of the eye area. They result from years of repetitive squinting and smiling,” said Dr. Steven Dayan, Founder of DeNova Research, Clinical Assistant Professor at the University of Illinois and a clinical investigator in the BOTOX® Cosmetic crow’s feet clinical trials. “I often see patients who are bothered by their crow’s feet lines, so I am very pleased that Allergan has conducted additional research to receive FDA approval of BOTOX® Cosmetic for this new indication. Based on the clinical evidence, I can now provide my patients with an FDA-approved option to address the crow’s feet lines that develop around the eyes.”
BOTOX® Cosmetic is a prescription medication that is injected into the muscles around the eye area to temporarily improve the look of moderate to severe crow’s feet lines in adults. It is a quick procedure that generally requires no downtime or recovery. BOTOX® Cosmetic works by blocking nerve impulses and reducing movement to the muscles around the eye area. The decreased muscle activity helps lesson the appearance of moderate to severe crow’s feet lines for noticeable results that do not radically change facial appearance or make a patient look as if they have had “work done.”
BOTOX® Cosmetic should be administered by a licensed, trained healthcare professional. Since 2002, more than 11 million treatment sessions for glabellar lines have been performed with BOTOX® Cosmetic. BOTOX®Cosmetic is approved in more than 75 countries for facial aesthetic use.
To learn more about BOTOX® Cosmetic, visit www.botoxcosmetic.com. Consumers can locate an authorized BOTOX® Cosmetic physician in their area by using the “find a doctor” tool located at www.botoxcosmetic.com.
BOTOX® Cosmetic (onabotulinumtoxinA) Important Information
Indications
BOTOX® Cosmetic is a prescription medicine that is injected into muscles and used to improve the look of moderate to severe frown lines between the eyebrows (glabellar lines) in adults for a short period of time (temporary).
BOTOX® Cosmetic is a prescription medicine that is injected into the area around the side of the eyes to improve the look of moderate to severe crow’s feet lines in adults for a short period of time (temporary).
IMPORTANT SAFETY INFORMATION
BOTOX® Cosmetic may cause serious side effects that can be life threatening. Call your doctor or get medical help right away if you have any of these problems any time (hours to weeks) after injection of BOTOX® Cosmetic:
- Problems swallowing, speaking, or breathing, due to weakening ofassociated muscles, can be severe and result in loss of life. You are at the highest risk if these problems are pre-existing before injection. Swallowing problems may last for several months.
- Spread of toxin effects. The effect of botulinum toxin may affect areas away from the injection site and cause serious symptoms including: loss of strength and all-over muscle weakness, double vision, blurred vision and drooping eyelids, hoarseness or change or loss of voice (dysphonia), trouble saying words clearly (dysarthria), loss of bladder control, trouble breathing, trouble swallowing. If this happens, do not drive a car, operate machinery, or do other dangerous activities.
The dose of BOTOX® Cosmetic is not the same as, or comparable to, any other botulinum toxin product.
There has not been a confirmed serious case of spread of toxin effect when BOTOX® Cosmetic has been used at the recommended dose to treat frown lines or crow’s feet lines.
Serious and/or immediate allergic reactions have been reported. They include: itching, rash, red itchy welts, wheezing, asthma symptoms, or dizziness or feeling faint. Tell your doctor or get medical help right away if you are wheezing or have asthma symptoms, or if you become dizzy or faint.
Do not take BOTOX® Cosmetic if you: are allergic to any of the ingredients in BOTOX® Cosmetic (see Medication Guide for ingredients); had an allergic reaction to any other botulinum toxin product such as Myobloc®(rimabotulinumtoxinB), Dysport® (abobotulinumtoxinA), or Xeomin®(incobotulinumtoxinA); have a skin infection at the planned injection site.
Tell your doctor about all your muscle or nerve conditions, such as amyotrophic lateral sclerosis (ALS or Lou Gehrig’s disease), myasthenia gravis, or Lambert-Eaton syndrome, as you may be at increased risk of serious side effects including severe dysphagia (difficulty swallowing) and respiratory compromise (difficulty breathing) from typical doses of BOTOX®Cosmetic.
Tell your doctor about all your medical conditions, including: plans to have surgery; had surgery on your face; weakness of forehead muscles, such as trouble raising your eyebrows; drooping eyelids; any other abnormal facial change; are pregnant or plan to become pregnant (it is not known if BOTOX® Cosmetic can harm your unborn baby); are breast-feeding or plan to breast-feed (it is not known if BOTOX® Cosmetic passes into breast milk).
Tell your doctor about all the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal products. Using BOTOX® Cosmetic with certain other medicines may cause serious side effects. Do not start any new medicines until you have told your doctor that you have received BOTOX® Cosmetic in the past.
Especially tell your doctor if you: have received any other botulinum toxin product in the last 4 months; have received injections of botulinum toxin, such as Myobloc®, Dysport®, or Xeomin® in the past (be sure your doctor knows exactly which product you received); have recently received an antibiotic by injection; take muscle relaxants; take an allergy or cold medicine; or take a sleep medicine.
Other side effects of BOTOX® Cosmetic include: dry mouth, discomfort or pain at the injection site, tiredness, headache, neck pain, and eye problems: double vision, blurred vision, decreased eyesight, drooping eyelids, swelling of your eyelids, and dry eyes.
For more information refer to the Medication Guide or talk with your doctor.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please see BOTOX® Cosmetic full Product Information including Boxed Warning and Medication Guide.
About Allergan
Allergan is a multi-specialty health care company established more than 60 years ago with a commitment to uncover the best of science and develop and deliver innovative and meaningful treatments to help people reach their life’s potential. Today, we have approximately 11,200 highly dedicated and talented employees, global marketing and sales capabilities with a presence in more than 100 countries, a rich and ever-evolving portfolio of pharmaceuticals, biologics, medical devices and over-the-counter consumer products, and state-of-the-art resources in R&D, manufacturing and safety surveillance that help millions of patients see more clearly, move more freely and express themselves more fully. From our beginnings as an eye care company to our focus today on several medical specialties, including eye care, neurosciences, medical aesthetics, medical dermatology, breast aesthetics, obesity intervention and urologics, Allergan is proud to celebrate more than 60 years of medical advances and proud to support the patients and customers who rely on our products and the employees and communities in which we live and work. For more information regardingAllergan, go to: www.allergan.com.
Forward-Looking Statements
This press release contains “forward-looking statements” including the statements by Dr. Scott Whitcup and Dr. Steven Dayan and other statements regarding the use of BOTOX® Cosmetic to treat crow’s feet lines. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or unknown risks or uncertainties materialize, actual results could vary materially from Allergan’s expectations and projections. Risks and uncertainties include, among other things, general industry and medical device market conditions; challenges related to achieving regulatory approval from the FDA on a timely and cost-efficient manner; technological advances and patents attained by competitors; inconsistency of treatment results among patients; potential difficulties in manufacturing; challenges related to new product marketing, such as the unpredictability or market acceptance for new products and/or the acceptance of new indications for such products; and governmental laws and regulations affecting domestic and foreign operations. Allerganexpressly disclaims any intent or obligation to update these forward-looking statements except as required by law. Additional information concerning these and other risks can be found in press releases issued by Allergan, as well as Allergan’s public filings with the U.S. Securities and Exchange Commission, including the discussion under the heading “Risk Factors” in Allergan’s most recent Annual Report on Form 10-K and any subsequent Quarterly Reports on Form 10-Q. Copies of Allergan’s press releases and additional information about Allergan are available at www.allergan.com or you can contact the Allergan Investor Relations Department by calling 1-714-246-4636.
©2013 Allergan, Inc. Irvine, CA 92612.
®marks owned by Allergan, Inc.
Allergan
Bonnie Jacobs (856) 912-9965; (714) 246-5134 (media);Jacobs_Bonnie@allergan.com
Lanie Shapero (732) 991-4368; (908) 203-2631 (media);Shapero_Lanie@allergan.com
Jim Hindman (714) 246-4636 (investors)
Joann Bradley (714) 246-4766 (investors)
David Nakasone (714) 246-6376 (investors)
Source: Allergan, Inc.
News Provided by Acquire Media